Ophthalmic
Single-use instruments
innovative instruments for ophthalmic surgery.

Enjoy the multiple benefits of unique features

Less carbon dioxide
Production-related carbon dioxide emissions halved.

Less risk
Less effort, always sterile.

Less resources
Less costs, more operations.
Only those who go off the beaten path can leave their own trail. “Single-use” and “sustainability” should really be mutually exclusive – right? 1stQ single-use instruments not only show that the opposite is true, but also that precision instruments of exceptional quality can be achieved with excellent value for money. In practice, single-use instruments also mean less effort is required for otherwise work-intensive processes such as collection, cleaning and sterilization. This is made possible by a sophisticated concept, an exclusive manufacturing process in Germany and the visionary foresight that distinguishes 1stQ.
Thanks to innovative production technology and a sophisticated recycling concept, 1stQ single-use instruments offer numerous advantages for users and patients alike.
Thomas Diehm, Managing Director Technology at 1stQ
From single-use instrument to value chain
1stQ single-use instruments (1) not only reflect our commitment to quality, but also our sense of responsibility. This is partly thanks to a unique and revolutionary recycling process in which our recycling partner recovers high-quality plastic granulate from used instruments, which can be used to manufacture new products. We are working to incorporate more and more waste products from ophthalmic surgery into a closed recycling loop and value chains in future, and thus make sustainability the industry standard.
The instruments are used once for a treatment and guarantee 100 % sterility.
Once used, single-use instruments are bagged and separated from other OR waste.
Our recycling partner collects and sorts the used single-use instruments by type.
Our recycling partner then processes the waste into high-quality regranulate.
The regranulate is reintroduced into the production cycle where it can be used to manufacture a wide variety of products (not yet suitable for medical devices).
Single-use instruments become the granular basis for creating new value





Significantly reduced CO2 footprint
According to a study conducted by the Fraunhofer Institute for Building Physics IBP, sustainability and single-use go together incredibly well:The screening study according to ISO 14044 that we commissioned verifies the excellent environmental footprint of 1stQ’s single-use products.
“The CO2 footprint of 1stQ single-use instruments with polymer handles is significantly lower than single-use instruments made entirely of steel. The 1stQ recycling concept further optimizes the CO2 footprint.”(2)
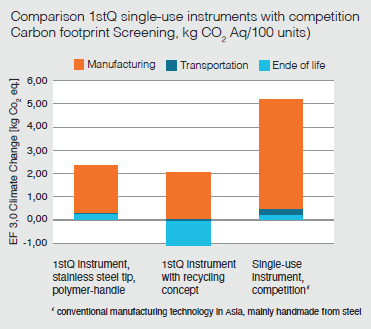
Fraunhofer Institute for Building Physics IBP, Stuttgart
July 3, 2020
Innovative production
1stQ single-use instruments are manufactured in Germany using a state-of-the-art production process: A high degree of automation guarantees a consistently high quality and maximum precision, while also making production more efficient. This, in turn, has a positive impact on resource consumption and production costs.
Our responsibility
As a commercial enterprise, partner of the health care sector and employer, we are obliged to make and implement well thought-out decisions.


“Creating added value through innovation”
With our single-use instruments, we are able to offer a more hygienic, more efficient and at the same time more sustainable solution – not despite, but because we have thought outside the box. After all, if you want to be innovative, you have to eliminate certain phrases from your vocabulary. For example: “We’ve always done it like this.”
Thomas Diehm
Managing Director Technology
For information about our SUIs, please contact your regional manager or:

Literature and references
(1)Development and production controlled by the 1stQ management.Legal manufacturer in accordance with the Medical Devices Directive/Regulation: Gemma Medical AG, 2555 Brügg, Mattenstraße 11, Switzerland. (www.gemma-medical.ch) (2) 1stQ, Techprotect, Screening of the carbon footprint for single-use medical instruments (SUI), Fraunhofer Institute for Building Physics IBP, life cycle assessment according to ISO 14040 and 14044. Data on file.

 IOL-Experience
IOL-Experience Technologies
Technologies IOL calculators
IOL calculators Service & handling
Service & handling Sustainability
Sustainability Congresses and events
Congresses and events Download Center
Download Center